Chemistry of an Oil Spill
This is part of an ongoing exploration of society's relationship with oil and the large part oil plays in all of our lives. AUG. 21, 2012 — Oil. For something that we all depend on every day, how much do we really understand about what it is and why it's so useful? Oil comes from beneath the ground. It is made of dead animal and plant matter, buried deep under layers of sedimentary rock. Pressure and heat cause oil deposits to form over long periods of time. But what is oil at its most basic?
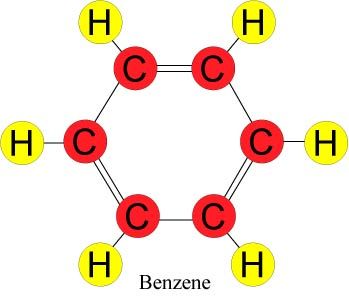
Oil is a complex mixture of molecular compounds. A molecule is the smallest unit of a substance that retains the substance's characteristics. Molecules, in turn, are composed of atoms. There are only 90 naturally occurring types of atoms on earth; these form the basis of the innumerable types of molecules found in nature. Crude oils, while mixtures of thousands of types of molecular compounds, are predominantly composed of only two types of atoms: hydrogen (H) and carbon (C). Molecular compounds composed exclusively of these two elements are called hydrocarbons. Petroleum hydrocarbons are predominantly one of two types, aromatics or alkanes. Aromatics, which are based on a 6-carbon ring, tend to be the molecular compounds in oil that are the most toxic to marine life.
A notable case is polycyclic aromatic hydrocarbons (PAHs), which have multiple carbon rings and can also be quite persistent in the environment. Alkanes, on the other hand, tend to be less toxic and are much more readily biodegraded naturally; most can be ingested as food by some microorganisms. For example, the oil spilled from the 2010 Deepwater Horizon/BP well blow-out was relatively high in alkanes and relatively low in PAHs. But, like all crude oils, it contained benzene, toluene, and xylene, which belong to the single-ring aromatic group. Benzene is very toxic and known to cause cancer but is not as persistent as PAHs.
Refining crude oil to produce fuel oils like gasoline and diesel does not significantly alter the molecular structure of the oil's components. So fuel oils usually contain the same types of molecular compounds that are found in their parent crude oils. Different chemical compounds can be extracted from crude oil and then recombined or altered to make what are called petrochemicals. Petrochemicals are used to make a vast array of products, including acetic acid, ammonia, polyvinyl chloride, polyethylene, lubricants, adhesives, agrochemicals, fragrances, food additives, packaging, paint, and pharmaceutical products. And that's just the start!

NOAA's Office of Response and Restoration is the primary science adviser to the U.S. Coast Guard during a major oil spill. Knowledge of the chemical make-up of the particular oil, whether it is a crude oil or refined fuel oil, is critical in making response decisions when there is spill. Among the scientists that work in OR&R's Emergency Response Division are chemists that are experts in this field. Crude oil is predominantly a mixture of hydrocarbons, but every crude oil is a unique mixture of molecular compounds. There are thousands of named crude oils in use around the world. Our chemists make recommendations by determining the source of the spill and the optimal cleanup methods and safety issues, based on the unique properties of the oil released. Next up, we'll delve into the toxicity of oil and the harm it can cause when accidentally released into the marine environment.
 An official website of the United States government.
An official website of the United States government.